NeuroLINCs : Non-invasive IVD test measuring brain-enriched lncRNAs specific to NDs.
LncRNAs are the most tissue-specific family of RNAs opening new horizons for diagnostic and therapeutic applications in the field of NDs. Identification of NeuroLINCs’ lncRNAs was performed using deep sequencing of human blood from patients with different stages of Alzheimer, patients with other dementia types, cognitively intact healthy controls and postmortem brains.
Expression levels were compared to select the brain enriched lncRNAs that are differentially expressed in the blood.
A total of 7494 lncRNAs, including 1816 novel lncRNAs never previously described, were identified and those which correlate with the relevant biomarkers of Alzheimer brain pathology (CSF Abeta or Tau) and neurodegeneration (volumetric scores of mediotemporal and hippocampal atrophy) are included into NeuroLINCs panel.
NeuroLINCs is a proprietary innovative IVD test developed for diagnostic applications for patients with a condition of cognitive impairment, Alzheimer’s disease and other neurodegenerative diseases. It measures brain-enriched and ND specific long non-coding RNAs (lncRNAs), identified and patented by Amoneta Diagnostics.
The selection of lncRNAs is based on projects and deep analysis of more than 20 billion of reads generated from postmortem brain tissue and peripheral blood samples; to detect with high accuracy patients with early Alzheimer’s disease or with other neurodegenerative diseases.
Detection and precise quantification of NeuroLINCs lncRNAs in blood is empowered by use of Celemics, Inc. specific probes to enrich selected lncRNAs for targeted sequencing on NGS platforms.
Features and benefits
First in class test
for the measurement of brain enriched lncRNAs in peripheral blood.
First test of blood lncRNA biomarkers
for diagnostic of MCI, early Alzheimer and other dementia types.
Investigation of a novel class
of therapeutic targets.
Proprietary list
of brain enriched lncRNAs.
More than 100.000 capture probes
for high coverage of lncRNA sequences.
Provides insights into brain condition
using blood, removing the need for brain biopsies.
NeuroLINCs provides first class results
Simplified workflow
Non-invasively collected samples to investigate lncRNAs.
PAXgene, plasma, brain tissue, PBMCs already validated.
Excellent analytical performance with high reproducibility of the results.
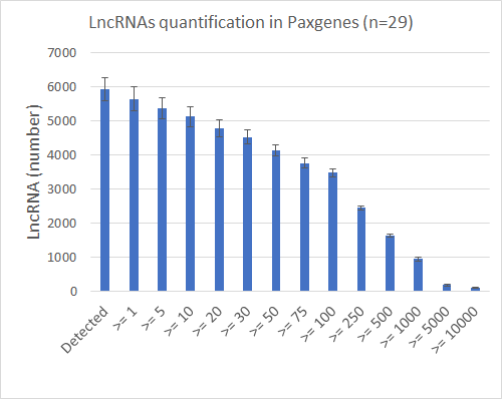
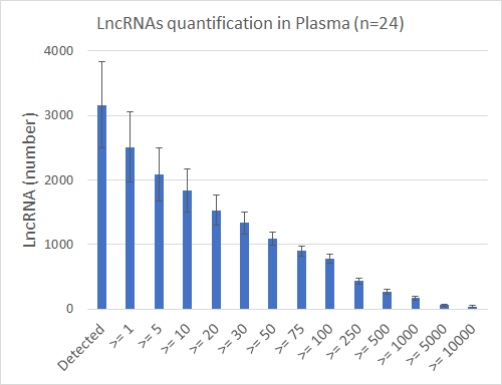
High sensitivity with 5930 lncRNAs detected in PAXgene and 3166 in Plasma over a total of 7494 lncRNAs.
Sample Preparation
Library Preparation
lncRNAs Capture
Sequencing
Data analysis


Optimize your biomarker projects and development programs
From concept to regulatory qualification, with significant experience in each step, we provide steadfast support across diverse areas
Biomarker design
Planning and study design
Development of SOPs
Regulatory support
Biomarker regulatory qualification
We are committed to improve disease outcome, therapeutic decisions and generate savings in healthcare
through biomarker discovery, development and regulatory qualification.

